The volume of information coursing through modern hospitals is expanding at an almost astronomical rate: analysts expect the world to generate more than 180 zettabytes of data next year, and more than one-third of it will originate in healthcare. Yet fully 97 percent of this data will never reach a clinician’s eyes, and six out of ten physicians now report spending more time in electronic medical records than with their patients—an imbalance that fuels burnout and delays care. GE HealthCare is addressing the problem head-on by embedding artificial intelligence capabilities at three critical layers of the system: inside imaging devices, across the patient journey, and throughout day-to-day hospital operations.
The company’s Bangalore- and Milwaukee-based Advanced Technology Group (ATG) has become the engine of that effort, translating fundamental research into commercial products at an impressive clip—twenty programs moved to market, more than 160 patents filed, and eighty peer-reviewed papers published in only five years. Below are six innovations that demonstrate how ATG solves fundamental technical hurdles once, and then scales each breakthrough so its benefits reach millions of patients.
1. AIRx Suite: Automating MRI Slice Prescription
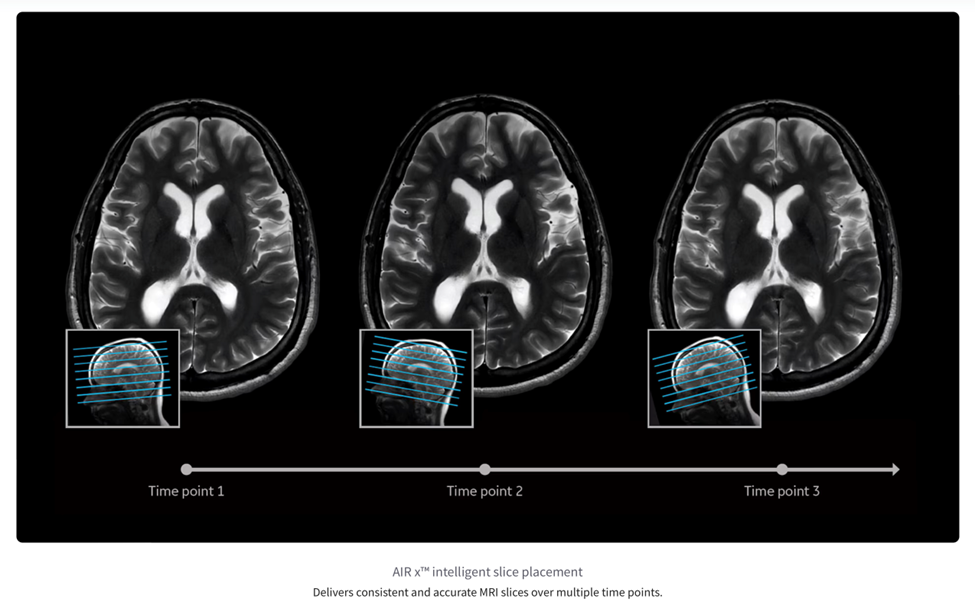
AIRx is a software tool that analyzes the three‑plane “localizer” scan taken at the very beginning of an MRI exam and then automatically chooses the MRI slices—thin, cross‑sectional pictures of the body—that an experienced technologist would normally set up by hand. The underlying deep-learning model powering AIRx was trained on tens of thousands of images, and detects patient anatomy with sufficient accuracy to hold slice-to-slice angular error below two degrees across neurological and knee protocols.
In practice this reduces a typical 95-click prescription workflow to just two confirmation clicks. Shorter table times ease claustrophobia for patients and free technologists to spend more time interacting with patients, while perfectly aligned follow-up studies give radiologists clear side-by-side comparisons that improve the detection of subtle disease progression.
2. True Enhance DL: Dual-Energy-Style Contrast on a Standard CT Scanner
Dual‑energy CT scanners capture two separate X‑ray spectra—two distinct energy levels—at the same time. This twin‑beam method improves iodine contrast—an iodine‑based dye that makes blood vessels and tumors stand out on the scan—for tumor staging and vascular imaging. However, it still relies on specialized detectors and high‑voltage generators that remain expensive. True Enhance DL circumvents that barrier by using a dedicated neural network to transform single-energy projection data into virtual 50 keV monochromatic images, thereby mimicking the visual impact of dual-energy acquisitions without exposing them to unnecessary additional radiation or hardware.
A white paper released in July 2023 describes four phase-specific models calibrated for angiographic, arterial, portal-venous, and delayed-phase imaging. The algorithm has now received U.S. FDA clearance and is available to new and existing Smart Subscription customers. By unlocking premium contrast performance on the scanners hospitals already own, the software broadens access to high-confidence oncology protocols and reduces the cost of care.

3. True Fidelity: High-Definition, Low-Dose CT Reconstruction
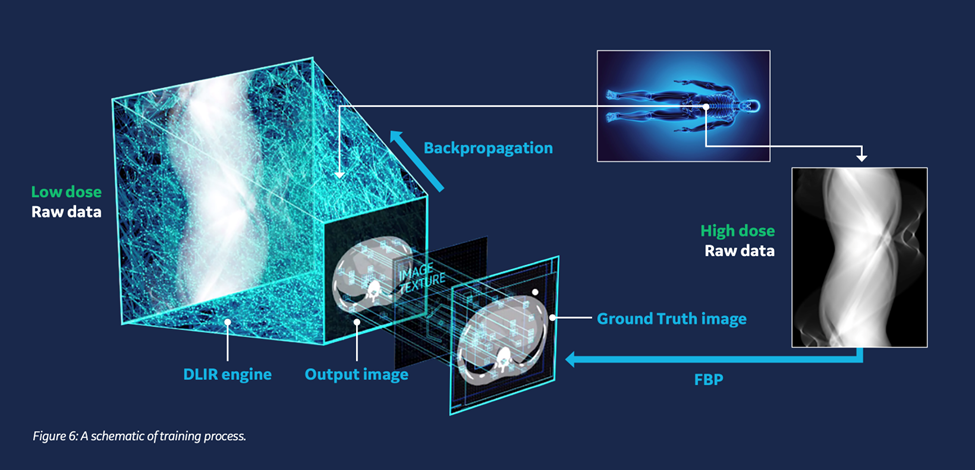
GE HealthCare’s True Fidelity deep-learning image-reconstruction engine was the first CT algorithm of its kind to receive U.S. FDA 510(k) clearance in November 2019. Trained on matched pairs of high‑ and ultra‑low‑dose scans, the network recognizes quantum noise—random speckles that appear when too few X‑ray photons are recorded—and removes it while preserving natural edge detail and image texture. A technical white paper summarizing subsequent multi-center studies reports dose reductions of up to 82 percent with either maintained or improved low-contrast detectability. Children, trauma victims, and oncology patients who require frequent imaging therefore receive diagnostically robust scans with a fraction of the radiation exposure historically required.
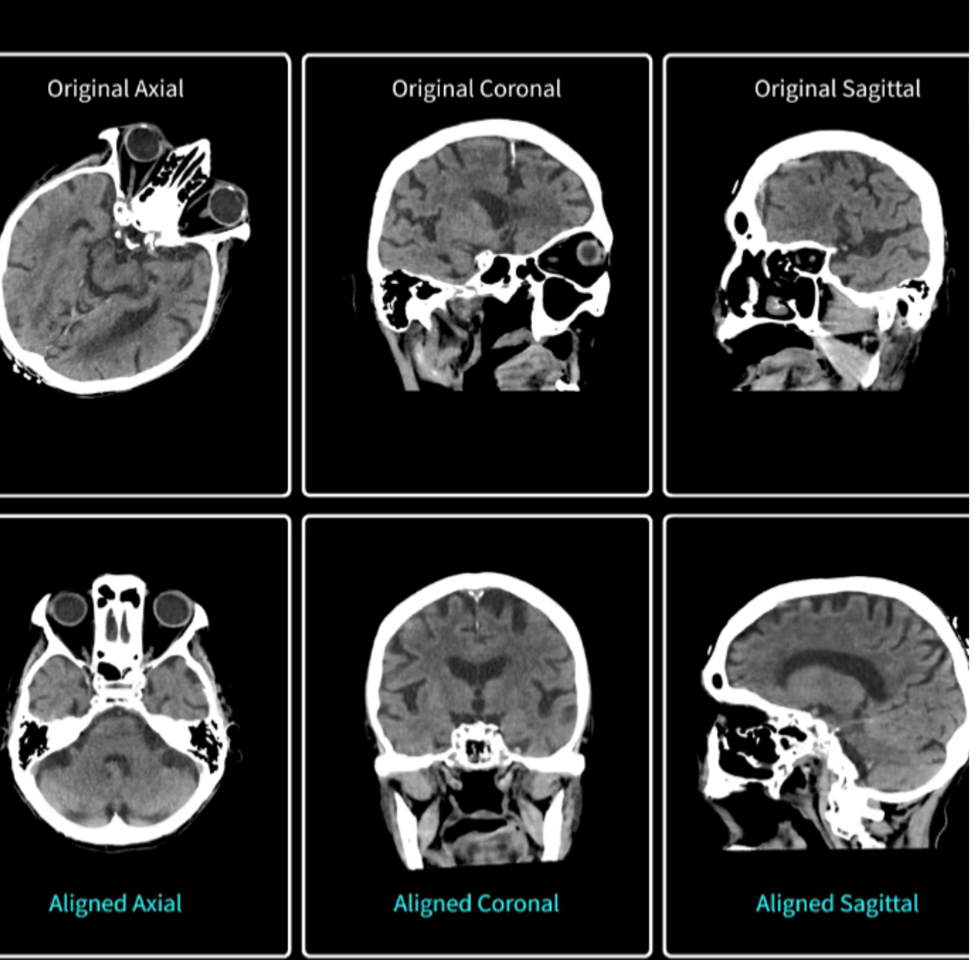
4. Head Auto-View: Ten-Second Cranial Reformats for Emergency Care
Post-processing a head CT traditionally involves rotating the image stack until the orbits, skull base, and foramen magnum align with canonical axial, coronal, and sagittal planes. This is a manual step that can add several minutes to a stroke or trauma workflow when time is critical. Head Auto-View automates the task by locating stable cranial landmarks and re-orienting the entire volume in roughly ten seconds.
Head Auto-View enables a 90 percent plus accuracy for patients scanned in the supine position, with automatic generation of axial, coronal, sagittal, and 3-D reformats routed directly to predetermined DICOM destinations. By eliminating a time-consuming bottleneck, the software can trim precious minutes from “door-to-needle” intervals when every minute of brain perfusion counts.
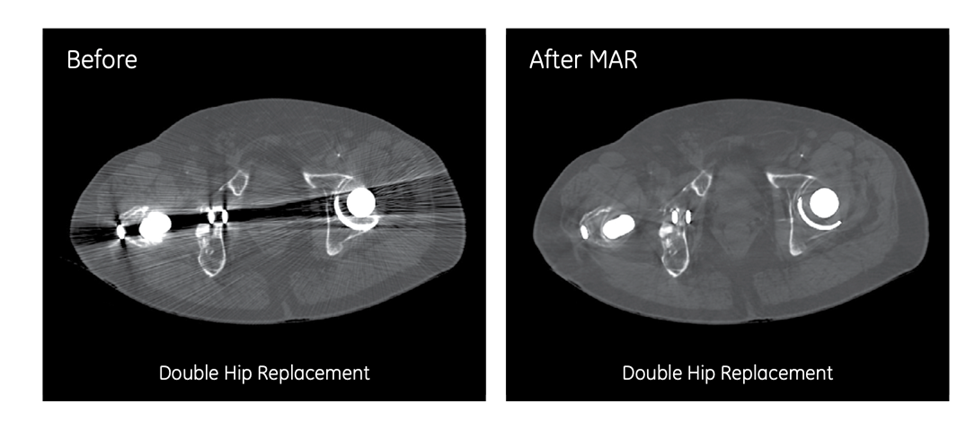
5. Metal-Artifact Synthesis: Inclusive MRI AI for Patients with Implants
Metal hardware in hips, spines, or knees generates complex susceptibility artifacts that confound many artificial intelligence models, largely because authentic implant cases are too scarce and heterogeneous to use in conventional training pipelines.
ATG’s metal-artefact-synthesis framework addresses the problem by segmenting real implants from 35 clinical knee and spine scans, converting those masks into digital templates, and then “blending” them onto routine images at random sizes, orientations, and intensities. When the augmented dataset was used to retrain a knee-image classifier, accuracy on scans containing implants rose from 79.7 percent to 88.5 percent. The approach ensures that roughly one in five MRI patients who carry permanent hardware can now benefit from AI-driven image triage and reporting with the same reliability as hardware-free patients.
6. Data-Diversity Scoring: Objective Cohort Selection for Deep Learning
Building ever-larger image banks does not automatically translate into better model performance. In many cases, labelling duplicate or near-duplicate studies diverts resources without adding information value.
Building bigger collections of medical images doesn’t automatically make AI models better. In many cases, labeling images that are duplicates or nearly the same takes up time and money without adding any real value. This becomes a problem when teams have limited resources and tight budgets.
To address this, ATG created a special dashboard that uses a method called UMAP. This tool looks at the hidden features in images and places them on a two-dimensional map. It then checks how far each new image is from existing groups of similar images. This helps identify which images are truly different and worth labeling.
In a trial run at a hospital that serves underrepresented communities, the system chose only the most unique and useful images. This improved the AI’s accuracy from 78% to 89%. By measuring how new or different each image is right from the start, the system helps teams spend their labeling budget on the most valuable data. This speeds up the safe use of AI in new areas and helps ensure the AI works fairly for all types of patients.
These six projects demonstrate a common engineering philosophy: solve a technically demanding problem and scale the benefit to propagate across the entire care continuum. Whether reducing MRI setup from 95 clicks to two, recreating dual-energy contrast on legacy CT scanners, or making AI robust to metallic implants, GE HealthCare’s Advanced Technology Group is methodically transforming artificial intelligence from a laboratory curiosity into a quiet—but pervasive—force for faster, safer, and more consistent patient care.