Published in: Journal of Clinical Oncology, Volume 43, Number 16_suppl
Abstract
Background: Immunotherapy (IO) is a viable therapeutic approach for non-small cell lung cancer (NSCLC). Despite the significant survival benefit of immune checkpoint inhibitors PD-1/PD-L1 in NSCLC, the objective response rate is not more than 50%, even with the combination of chemotherapy. There is heterogeneity in response to IO with patients with metastatic disease with long-term response, lasting years, and on the other hand, patients who fail to respond at all. While PD-L1 is a known biomarker to stratify IO responses, it is, at best, imperfect. There is thus an urgent need to discover new predictive multimodal biomarkers of response to IO in such a setting.
Methods: A retrospective cohort of 187 patients (Memorial Sloan Kettering – MSKCC MIND cohort, training=140, validation=47) with advanced NSCLC, treated with PD-L1 blockade, were analyzed to develop a machine learning-based algorithm predictive of response to immunotherapy. Multimodal baseline data, including clinical parameters, PD-L1 expression, TMB, and baseline CT scan data, were used to predict treatment response status (responders vs. non-responders). For each patient, available tumor masks were used to generate peritumoral masks. 1st, 2nd, and higher-order statistics of extracted 8 Haralick radiomics textures and intensity gradients of intratumoral and peritumoral areas were used. A 2-step feature selection was performed, optimizing Gini impurity and Area Under the Curve (AUC) with Random Forest (RF) to select the best intratumoral and peritumoral radiomic features and their respective probabilities of predicting response in the training cohort using 5-fold cross-validation. These radiomics-based prediction probabilities were combined with clinical data like smoking status, age, TMB, and PD-L1 score to train another RF model to predict response. They were validated by computing the AUC of the ROC on the validation cohort.
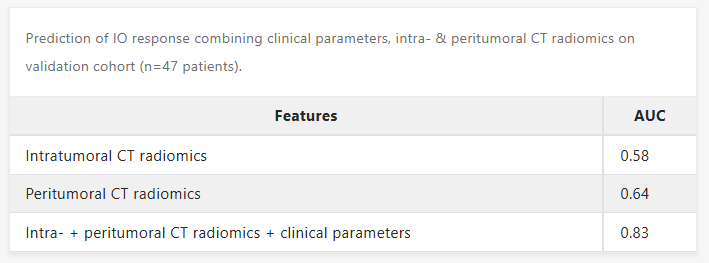
Results: The combination of clinical, intratumoral, and peritumoral features led to an impressive prediction of response to IO in NSCLC patients from our database, with an AUC=0.83. Peritumoral radiomics features alone led to an AUC=0.64 compared to intratumoral radiomics features of AUC=0.58, suggesting the importance of tumor microenvironment in the surrounding tumor areas in prediction of IO response.
Conclusions: This proof-of-concept study suggests that machine learning applied to baseline multimodal data, including CT tumoral radiomics-based prediction probabilities and clinical variables, can help predict response to IO and improve patient selection.